CLINICAL TRIALS
April clinical trial for acute ischemic stroke
The APRIL clinical trial was a Phase 1b/2a multicentre, randomised, double-blind and placebo-controlled study that evaluated the safety and primary efficacy of our lead neuroprotector drug ApTOLL for acute ischemic stroke.
The administration of ApTOLL in combination with endovascular therapy (EVT) yielded groundbreaking results in Phase 1b/2a in patients: reduced mortality of patients from 18% to 5% compared to the placebo group, imaging tests given 72 hours after treatment showed that the size of damaged brain tissue was reduced by 50% among the patients who received the higher dose of ApTOLL compared to the placebo group, and 64% of patients who received the higher dose of ApTOLL were free of disability at 90 days compared to 47% of placebo.
The data was presented at the opening session of the 2023 International Stroke Conference (ISC) in Dallas, United States, and published at JAMA Neurology and Frontiers in Neurology.
For additional information, visit Clinicaltrials.gov.
Scientific publications
Changes in Infarct Volume and Cerebral Edema After Mechanical Thrombectomy in Patients With Stroke Randomized to ApTOLL or Placebo: A Secondary Analysis of April Trial
Stroke (2025)
Hernández-Pérez M, Valls-Carbó A, Hernández-Jiménez M, Molina C, Vivancos Mora J, Castellanos M, Masjuan J, Tembl J, Moniche F, Terceño M, Cardona P, Arenillas J, Calleja P, Henon H, Calviere L, Mazighi M, Olivot J, Liebeskind J, Ribo M.
Clinical trial to compare safety and tolerability between intravenous infusion and bolus intravenous injection of ApTOLL in healthy volunteers
Molecular Therapy Nucleic Acids (2024)
Hernández-Jiménez, M; Martín-Vílchez, S; Mejía-Abril, G; Roman M; Luquero-Bueno, S; Piñeiro, D; Ribó, M; Abad-Santos, F; Ochoa, D.
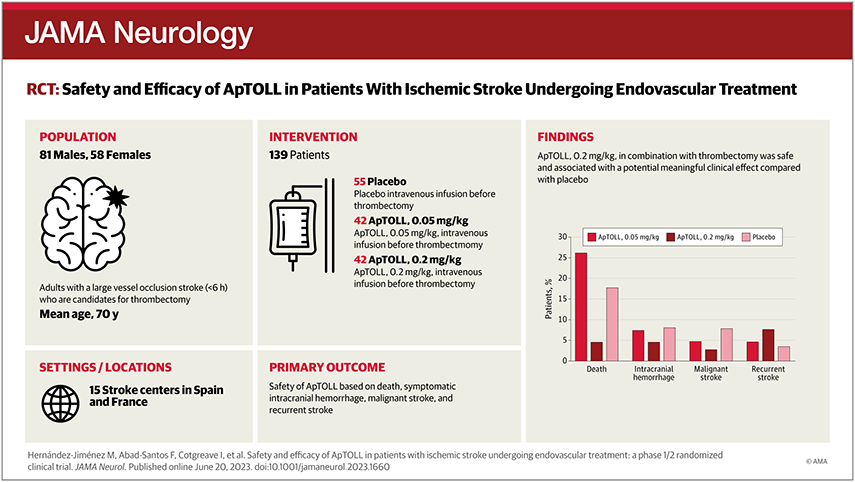
Safety and Efficacy of ApTOLL in Patients With Ischemic Stroke Undergoing Endovascular Treatment. A Phase 1/2 Randomized Clinical Trial
JAMA Neurology (2023)
Hernández-Jiménez, M; Abad-Santos, F; Cotgreave, I; Gallego, J; Jilma, B; Flores, A; Jovin, T; Vivancos, J; Hernández-Pérez, M; Molina, C; Montaner, J; Casariego, J; Dalsgaard, M; Liebeskind, D; Cobo, E; Castellanos, M; Cardona Portela, P; Masjuán, J; Moniche, F; Tembl, J; Terceño Izaga, M; Arenillas, J; Calleja, P; Olivot, J; Calviere, L; Henon, H; Mazighi, M; Piñeiro, D; Pugliese, M; González, V; Moro, M; Garcia-Tornel, A; and Ribó, M.
APRIL: A double-blind, placebo-controlled, randomized, Phase Ib/IIa clinical study of ApTOLL for the treatment of acute ischemic stroke
Frontiers in Neurology (2023)
Hernández-Jiménez, M; Abad-Santos, F; Cotgreave, I; Gallego, J; Jilma, B; Flores, A; Jovin, T; Vivancos, J; Molina, C; Montaner, J; Casariego, J; Dalsgaard, M; Hernández-Pérez, M; Liebeskind, D; Cobo, E; and Ribo, M.
First-in-human phase I clinical trial of a TLR4-binding DNA aptamer, ApTOLL: Safety and pharmacokinetics in healthy volunteers
Molecular Therapy Nucleic Acids (2022)
Hernández-Jiménez, M; Martín-Vílchez, S; Ochoa, D; Mejía-Abril, G; Román, M; Camargo-Mamani, P; Luquero-Bueno, S; Jilma, B; Moro, A; Fernández, G; Piñeiro, D; Ribó, M; González, V; Lizasoain, I; Abad-Santos, F.
